Reference: Beginning Physics II
Chapter 17: PARTICLES OF LIGHT AND WAVES OF MATTER
.
KEY WORD LIST
Localization, Interference, Energy Flow, Quantization, Light, Black Body Radiation, Photo-Electric Effect, Production of X-rays, Compton Scattering, Matter Waves, Probability Distribution, Maxwell’s Wave Equation, Schrödinger’s Equation, Dirac’s Equation, Wave Function, Wave Packet, Uncertainty Principle, Zero Point Energy, Tunnelling.
(From KHTK) Particle, Quantum, Motion.
.
GLOSSARY
For details on the following concepts, please consult Chapter 17.
LOCALIZATION
A particle is defined as a “point”. The properties of velocity and acceleration are attributed to the whole particle. There is certainly no possibility of considering any one particle as being simultaneously at many different positions in space. A wave is defined as a disturbance that is spread over a fairly sizable area. A pure sinusoidal wave is spread out from plus to minus infinity. A pulse wave has a definite extent in space at any instance. One generally thinks of a wave as being a disturbance located simultaneously at many points of space. Therefore, the particle and wave picture differ on the question of localization.
INTERFERENCE
The interference or diffraction phenomenon is observed with waves but not with particles.
ENERGY FLOW
Both waves and particles carry energy and momentum. But there is a big difference in the mechanism of that energy flow. A wave carries this energy in a continuous fashion without discontinuous jumps in energy flow, even if the energy is greatly decreased. However, in case of particles, the energy comes in discontinuous pulses, with each pulse bringing a discrete amount of energy. The flow of energy is not uniform or continuous. This difference leads to the idea of quantization.
QUANTIZATION
In case of light, a continuously flowing wave seems to transport quantized units of energy.
LIGHT
Although the experiments on interference show that light behaves as a wave, the photo-electric effect provides the most convincing evidence that light must be considered to consist of particles. This apparent anomaly between wave and particle nature is resolved in the definitions from KHTK provided below.
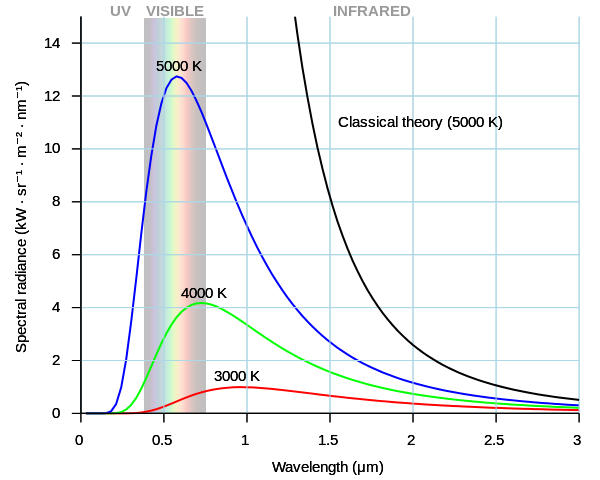
BLACK BODY RADIATION
This radiation is emitted over a continuous spectrum of frequencies. It increases rapidly at lower frequencies reaching a maximum and then starts to decline. Maxwell equations explain the distribution only at lower frequencies when it is increasing rapidly. They are unable to explain the rest of the distribution. But the whole distribution can be explained when energy is seen to be radiating in quanta that are proportional to frequency.
PHOTO-ELECTRIC EFFECT
In the photo-electric effect, a beam of light is shone on a metal, with the result that electrons are emitted from the metal. However, the maximum kinetic energy of the electrons emitted is found to depend on the frequency of the incident light and not on its intensity. This means that light energy has to be absorbed in quanta in the interaction that frees the electrons.
PRODUCTION OF X-RAYS
X-rays can be produced by taking energetic electrons and letting them strike a metal plate. When these electrons stop in the metal, they emit electromagnetic radiation over a large range of wavelengths. The surprising experimental fact is that there is a minimum wavelength that can be emitted for each V (energy of the electrons). This minimum wavelength varies inversely with V but is not affected by the electron current density. These results cannot be explained by a wave theory of light.
COMPTON SCATTERING
The X-ray beam, after it is scattered by electrons, suffers a definite reduction in frequency. Compton showed that energy of the photon, as given by its frequency, is reduced by the same amount that the kinetic energy of the recoil electron is increased. Thus, the photon is a momentum carrying corpuscle that can transfer its momentum in a given direction to the atom. The Compton effect also implies that the electron must be treated as a wave and not as a particle.
MATTER WAVES
To conform with the case of electromagnetic waves, De Broglie hypothesized that the frequency and wavelength for electrons should be determined by the same basic relations used for photons. If De Broglie’s hypothesis is true, then the electrons represented by these waves should exhibit interference and diffraction appropriate to the wavelengths associated with the electron. When experiments were performed using crystals as diffraction gratings, this diffraction was indeed seen. Similar predictions for the wavelengths of more massive particles, such as protons and neutrons, have also been experimentally verified. Thus, at small scales, there is dualism in nature between waves and particles. The velocity of the particles is equal to the group velocity of its associated wave. Planck showed that energy is connected to frequency, E = hf. De Broglie then showed that momentum is connected to wavelength, p = h/λ. This is a fundamental relation of the Quantum Theory.
PROBABILITY DISTRIBUTION
The interference of waves in the double slit experiment creates a distribution of the intensity of light. Quantum mechanics interprets this as a probability distribution of “point” particles called photons. This concept, that the only thing that we can predict is the probability of a photon’s location, is a basic concept of quantum mechanics.
MAXWELL’S WAVE EQUATION
Thus in the case of a photon, Maxwell’s wave equation gives the probability amplitude for finding a photon. This equation has to be solved to predict the behavior of the photon.
SCHRÖDINGER’S EQUATION
This is a similar equation that provides the probability amplitude for finding a non-relativistic electron.
DIRAC’S EQUATION
This equation provides the probability amplitude for finding a relativistic electron.
WAVE FUNCTION
The central idea is that there is some wave equation which has to be solved to predict the behavior of a particle. When this equation is solved for a particular case, the resulting wave (called a wave function) gives an amplitude which, when squared, is proportional to the probability distribution of the particles.
WAVE PACKET
Pulses of waves can be obtained by superposing a large number of regular traveling waves of different wavelengths. Such a pulse is called a “wave packet.” Such a packet traveling through space indeed resembles a localized particle.
UNCERTAINTY PRINCIPLE
The uncertainty principle states that if one wants to describe a particle (at a given time) as being localized in a region ∆x (i.e. have a spatial uncertainty ∆x), then that particle must have an uncertainty in its x direction momentum, ∆p, which is at least as large as h/∆x. Similarly, if one wants the x direction momentum to be known to within ∆p, then there must be an uncertainty in the position of the particle which is at least as large as ∆x = h/∆p. Of course, if p is uncertain, so is v and the kinetic energy.
ZERO POINT ENERGY
This means that particles of matter, even if the temperature is at absolute zero where there is no thermal energy, must still have an average kinetic energy related to this range of momenta. This kinetic energy at a temperature of absolute zero, is called the “zero-point energy”, and there is no way to avoid having this minimum amount of energy.
TUNNELLING
Energy uncertainty of a particle allows it to surmount the barrier from the inside to the outside of the well for the short time needed to travel and escape from the well. This process is called tunneling, since the particle appears to have dug a hole through the wall of the well and emerged on the outside. This process actually occurs in the radioactive decay of nuclei.
.
The following definitions are from the philosophy of KHTK:
PARTICLE
A particle is not a “point,” but it has a volume that is filled with substance. The substance within a particle has uniform consistency throughout. The more condensed is the substance, the smaller is the size of the particle. This predicts the relative sizes of a proton and an electron in a hydrogen atom. The size of the electron is equivalent to the size of the hydrogen atom. On the other hand, the size of the proton is roughly 2000 times smaller because its substance is that much condensed. This provides a proper visualization of the hydrogen atom, where an extremely small proton exists at the center of the only electron there is. A very condensed particle, like proton, may appear to be spherical in shape; but as the consistency of substance decreases, as in the electron, the particle is likely to expand in size and flatten into the shape of a disk.
QUANTUM
This quantum is the amount of energy involved in the process of radiating and getting absorbed. It does not necessarily mean that this energy exists in space in a pulse form. In space it may simply appear as a certain consistency (a degree of condensation) of energy.
MOTION
When the difference in condensations is very large, the condensed substance may appear as a particle next to the uncondensed substance. But if the difference in condensation is comparable, the two substances may appear as waves when set next to each other. Therefore, ‘wave’ and ‘particle’ is a matter of looking at substance at different scale of condensation. If you greatly magnify a particle, you may see waves inside it as a disturbance. The wave inside a particle may have a ‘motion’ commensurate with the condensation of its substance. This may appear to give the particle its relative speed compared to another particle.
We find that a slight change in the condensation of substance creates a huge change in the relative speed of its particle. This may be the leverage that thought has over physical movement. All animation in the body is most likely produced by infinitesimal shifts in condensations of substance. This principle may also underlie in controlling the speed and direction of the UFOs. The change in condensation must apply equally to the occupants of the UFO for intense accelerations not to be felt by them.
.