Reference: Propagation of Light
When space is disturbed electric and magnetic fields are generated. These fields oscillate as a transverse wave. The disturbance travels in a straight line at the speed of light.
Disturbance is manifested in space as oscillating electric and magnetic fields.
In each oscillation the disturbance advances by a distance called wavelength. The oscillations occur at a certain frequency. The speed of propagation is then determined by the product of frequency and wavelength. Oscillations are the result of a rotating vector. The propagation of disturbance may be compared to the advance of a rotating screw.
The disturbance propagates like a rotating vector threading into the space.
The space does not provide any resistance. The disturbance contains its own inertia through the interaction between electric and magnetic fields. This inertia resists any change to frequency and wavelength, and keeps the disturbance going.
The disturbance consists of its own inertia that sustains it.
The disturbance has a large range of frequencies and wavelengths. This range is represented by the electromagnetic spectrum consisting of radio waves, microwaves, infra-red light, visible light, ultra-violet light, X-rays and Gamma rays. This spectrum presents increasing frequency and shortening wavelengths, while the speed of propagation remains practically the same.
The disturbance forms a spectrum of increasing frequency and shortening wavelengths.
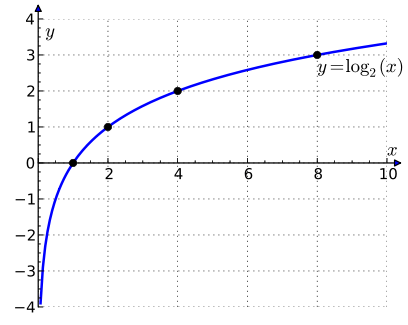
Since the spectrum extends over a very large range of frequencies, it may be managed more conveniently as Disturbance Levels on a logarithmic scale of base 2 (similar to octaves).
Disturbance Level Frequency
0 20 or 1
1 21 or 2
2 22 or 4
3 23 or 8
… …
n 2n
The disturbance levels of some of the electromagnetic frequencies are as follows
EM Frequency Disturbance Level
Visible light ………………….. ~ 49
Gamma Rays ………………… ~ 65
Electron ………………………. 66.7
Proton ………………………… 77.6
Neutron ……………………….. 77.6
Inertia seems to increase with increasing disturbance levels. The disturbance levels may provide a measure of inertia.
x represents frequency; y represents Disturbance Levels
These disturbance levels may be plotted as above. It can be seen from this graph that negative disturbance levels may be postulated to exist with the halving of frequency. The frequency never reaches zero, except theoretically. We may postulate a level of zero frequency to describe undisturbed space. This space forms the background of all disturbance. The undisturbed space has zero inertia.
The background of all phenomena is undisturbed space of zero frequency and no inertia.
The disturbance levels express inertia. When disturbance level crosses a threshold, the inertia transitions from “wave-disturbance” to “particle-mass.” Inside the atom, this transition occurs at the boundary of the nucleus.
The concept of “Disturbance Level” shall be used in subsequent discussions.
.


Comments
This Disturbance Model provides an inertial basis for all phenomena.
I shall continue commenting from Isaac Asimov’s UNDERSTANDING PHYSICS, Volume 3.
Click to access phys3.pdf
.
Asimov: “In one bound, the atoms, which from the time of Democritus on had been assumed to be the smallest particle of matter, were suddenly rendered giants. Here was something much smaller than even the smallest atom; something so small indeed that it could easily be visualized as worming its way through the interstices among the atoms of ordinary matter. That seemed one reasonable explanation for the fact that cathode rays made up of particles could penetrate thin sheets of metal. It also explained why electric currents could be made to flow through copper wires.
“Thomson, therefore, had not only discovered the electron, he had also discovered the first of the subatomic particles, and opened a new realm of smallness beyond the atom.”
.
Asimov: “Again the same problem arose. When light forced electrons out of an electrically-neutral metallic surface, were those electrons formed as they were emitted or did they exist within the metal at all times? By 1901, Einstein had shown that the law of conservation of mass was incomplete in the form that had generally been accepted during the nineteenth century. He showed that energy and mass could be interconverted and that one ought to speak of the law of conservation of mass-energy. Nevertheless, the book-keeping involved in the interconversion of mass and energy was rigorous, and there was insufficient energy in ordinary light–and even in ultraviolet light–to serve the purpose of manufacturing electrons.”
.
Electrons could have been formed as they were emitted. Mass from metal could have supplied the mass for the formation of the electrons. Electrons were not necessary formed from incident light.
.
Asimov: “Electrons, then, must exist in the metal at all times, and one could ask another question. Did the electrons exist in the interstices between the atoms, or did they actually occur within the atoms themselves? It was hard to accept the latter view, for that would mean that the atom was not the featureless, ultimately indivisible object that Democritus and Dalton had proposed and that the scientific world had finally accepted.”
.
Did electrons exist as “electrons” within the metal?
.
Asimov: “Philipp Lenard had observed that the energy with which electrons were ejected depended on the frequency of the light, and that light of less than a certain frequency, (the threshold value) did not eject electrons. The quantum theory which was beginning to come into acceptance in the first decade of the twentieth century, made it clear that light consisted of photons that increased in energy content as frequency increased.
The threshold value represented quanta of just sufficient energy to break the bonds holding the electrons to matter. The strength of those bonds varies from substance to substance, since electrons are forced out of some metals only by energetic ultra- violet light, whereas they are forced out of other metals by tight as un-energetic as the visible red. If electrons are tied to matter, it must be to the atoms they are bound, and with differing bond strengths, so to speak, depending on the nature of the particular atom. It seems only sensible to consider something always present near the atom, always bound to the atom with a characteristic force, to be part of the atom.”
.
It is being assumed here that electrons exist as “electrons” within the atom.
.
Asimov: “Furthermore, once the view is accepted, there are advantages to it. There are many varieties of atom and only one type of electron (since the particles emitted from all metals by the photo-electric effect are of identical properties). Perhaps the troublesome variety of the atoms could be explained in terms of the number of electrons each contained, of their arrangement, of the strength with which they were held, and so on. Perhaps the order enforced empirically upon the elements by the periodic table could now be made more systematic. If so, the indivisible atom of Democritus was well lost.”
.
It is also being assumed that atom is a solid particle. Could it be that the periphery of atom is made up of captured electromagnetic waves, which, when scooped up, formed electrons?
This also shows that an electron has a stable structure as a particle made up of “soft” inertial material of electromagnetic waves. It can be created easily from the electromagnetic waves scooped up from the periphery of the atom.
It would be interesting to investigate the structure of an electron.
.
Asimov: “Indeed, there were some facets of the photoelectric effect that fit in well with the periodic table. For instance, the elements that most readily give up electrons in response to light are the alkali metals. These give up electrons with increasing ease as atomic weight goes up that is, as one moves down the column in the periodic table. Thus cesium, the naturally-occurring alkali metal with the highest atomic weight,· releases its electrons most easily of all–hence Zworykin’s use of the metal in his iconoscope.”
.
The peripheral disturbance in atoms of alkali metals is likely to be scooped up more easily as their atomic weight goes up, because as atoms grow larger in size, the periphery is farther from the center.
.
Asimov: “The apparent existence of electrons within the atom raised some important questions. The atoms were electrically neutral; if negatively-charged electrons existed about or within the atom, there had to be a positive charge somewhere to neutralize the negative charge of the electrons- If so, where was it? Why didn’t light ever bring about the ejection of very light positively-charged particles? Why were there only cathode rays, never analogous anode rays?”
.
This raises a question regarding the nature of charge. Maybe the charge comes into play only when electron is formed the peripheral disturbance in the atom. There is no charge in a neutral atom, may be not even in some balanced form.
.
Asimov: “Thomson offered an answer to these questions. In 1898, he suggested that the atom was a solid, positively-charged sphere into which just enough electrons were embedded (like raisins in pound cake, so to speak) to bring about an overall electrical neutrality.”
.
Thomson assumed that electrons exist within an atom as “electrons.”
.
Asimov: “Thomson’s theory, although so attractive, nevertheless, had a fatal shortcoming Lenard had noted that cathode rays could pass through small thicknesses of matter To be sure; the electrons making up the cathode rays were very small and might be pictured as worming their way between the atoms. If so, they would most likely emerge badly scattered. Instead, cathode rays passed through small thicknesses of matter still traveling in an essentially parallel beam, as though they had passed through atoms without very much interference.
“In 1903, therefore, Lenard suggested that the atom was not a solid mass but was rather mostly empty space. The atom, in his view, consisted of tiny electrons and equivalent particles of positive charge, existing in pairs so that the atom as a whole was electrically neutral.”
“But, in that case, why were there only cathode rays and never anode rays?
“The reconciliation of the Thomson and Lenard views fell to the lot of the New Zealand-born physicist Ernest Rutherford (1871-1937). Beginning in 1906, he conducted crucial experiments in which he bombarded thin gold leaf with alpha particles. Behind the gold leaf was a photographic plate.
“The stream of alpha particles passed right through the gold leaf as though it were not there and fogged the photographic plate behind it. The gold leaf was only 1 /50,000 of a centimeter thick, but this still meant a thickness of 20,000 atoms. The fact that alpha particles could pass through 20,000 gold atoms as though they weren’t there was strongly in favor of Lenard’s notion of an empty atom, (an atom, that is, made up of nothing more than a scattering of light particles).”
.
Most of the atom seems to be made up of electromagnetic disturbance circling around a center. This disturbance is looser than an alpha particle or even a compact electron.
.
Asimov: “But the truly interesting point was that not all the alpha particles passed through unaffected. The spot of fogging on the plate would, in the absence of the gold leaf, have been sharp; but with the gold leaf in place, the boundary of the fogged spot was rather diffuse, fading out gradually. It was as though some-of the alpha particles were, after all, slightly deflected from their path. In fact, Rutherford was able to show that some were deflected more than slightly! About one alpha particle out of every 8000 was deflected through a right angle or even more.
…
By 1911, Rutherford was ready to describe his picture of the atom. In his view, Thomson’s massive positively-charged atom was still there as far as mass was concerned, but it was drastically shrunken in volume. It had shrunk down to an extremely small object in the very center of the atom. This massive central object was the atomic nucleus, and what Rutherford was proposing was the nuclear atom, a concept that has remained valid ever since and that is more firmly accepted now than ever.”
.
Asimov: “The atomic nucleus, as could be seen from the pattern of deflections of alpha particles, was tiny indeed, not more than 10-13 to 10-12 centimeters in diameter, or only 1/100,000 to 1/10,000 the diameter of the atom as a whole. The volumes of nucleus and atom are in proportion to the cube of the diameter, so the volume of the nucleus is rather less than one trillionth (1/1,000,000,000,000) of the atom as a whole.
…
Outside the nucleus, the comparatively vast remainder of the atom is made up of nothing but the ultra-light electrons. These electrons offer little obstacle to speeding cathode ray particles and virtually no obstacle at all to alpha particles; consequently, Rutherford’s nuclear atom is as thoroughly empty as Lenard’s model was.”
.
It is interesting that nucleus is assumed to be surrounded by electrons, rather than by an electron producing region.
.
Asimov: “When Rontgen first discovered X rays, he had produced them as a result of the impact of cathode ray particles on the glass at the end of the cathode-ray tube. Speeding electrons can penetrate small thicknesses of matter, but they are slowed down; if the obstructing matter is thick enough, they are stopped completely and absorbed. The deceleration of electrically charged particles will, according to Maxwell’s theory of electromagnetism result in the production of electromagnetic radiation, and this does indeed appear in the form of X rays.”
.
Asimov: “In 1911, Barkla showed that among the X rays produced at a given anticathode, certain groups pre- dominated. He could only judge the difference among the X-ray groups produced by their ability to penetrate thicknesses of matter …
The hardness of these sets of characteristic X rays varies with the nature of the metal making up the anticathode. In general, the higher the atomic weight of the metal, the harder the X rays produced…”
.
Asimov: “Moseley worked with the K-series of characteristic X rays for about a dozen consecutive elements in the periodic table, from calcium to zinc, and found that the wavelength of the X-rays went down (and the frequency therefore went up) as the atomic weight increased. By taking the square root of the frequency, he found that there was a constant increase as one went from one element to the next.
Moseley decided that there was something about the atom, which increased by regular steps as one went up the periodic table. It was possible to demonstrate that this “something” was most likely the positive charge on the nucleus. The most straightforward conclusion Moseley could reach was that the simplest atom had a charge of +1 on its nucleus; the next, a charge of +2; the next, a charge of +3, and so on. Moseley called the size of this charge the atomic number.”
.
The charge on the nucleus seems to be determined indirectly from the frequency of the X-rays emitted from those atoms.
.
Asimov: “Once the nuclear charge of an element was known, something was also known about the number of electrons in the atoms of that element, An element might lose an electron or two, or gain an electron or two, and become an electrically charged ion but in the neutral atom, the number of electrons had to be precisely enough to neutralize the nuclear charge. If, in the oxygen atom, the nucleus has a charge of +8, there must be eight electrons (each with a charge of -1) to balance that. We may say then, that the number of electrons in a neutral atom is equal to the atomic number of the element. The neutral hydrogen atom possesses 1 electron, the neutral sodium atom possesses 11 electrons and the neutral uranium atom possesses 92 electrons.”
.
Asimov: “After Thomson’s model had been abandoned and replaced by Rutherford’s nuclear atom, it remained possible that the electrons possessed some regular arrangement outside the nucleus. This notion seemed to be backed by the several series of characteristic X rays produced by various elements. Perhaps each series was produced by a separate group of electrons enclosing the central nucleus. The group nearest the nucleus would be most firmly held and would produce the hardest X rays, the K-series. The next group would produce the L-series, and so on. If the electrons were pictured as arranged spherically about the nucleus (like the shells making up an onion), one could speak of the K-shell, the L-shell, the M-shell, and so on, as one worked outward from the nucleus.”
.
It appears that the disturbance surrounding the nucleus increases in its “disturbance levels” as it gets closer to the center of the atom.
.
Asimov: “Perhaps, if the inert gases do not easily engage in chemical reactions, this is because their atoms already possess a particularly stable arrangement of electrons and have only the most minor tendency to upset that arrangement by indulging in the loss or gain of electrons. It seemed logical to suppose that this stable arrangement is represented by the complete filling of a particular shell of electrons.”
.
The disturbance around the nucleus shall be of spherical shape. This disturbance has a frequency and a wavelength. To be stable, the frequency should be such that wavelengths fit in their spherical shell as integers.
.
Asimov: “Soon after Moseley’s work, an attempt was made to rationalize the chemical reactions on the basis of electron distributions inside the atom. A relatively successful attempt was made, independently, by the American chemists Gilbert Newton Lewis (1875-1946) and Irving Langmuir (1881-1957). The essence of their views was that in any chemical reaction an element gained or lost electrons in such a way as to gain an “inert-gas configuration” that being the most stable arrangement.
…
“Indeed, the Lewis-Langmuir picture of electrons being transferred and shared has turned out to be a very useful way of picturing how the molecules of a great many of the simpler chemical compounds are held together.”
.
The above picture is useful as a metaphor, but the actual picture still needs to be worked out.
.