Reference: Essays on Substance
The Quantum
A quantum is a particle of substance, where substance is anything substantial enough to be sensed. A quantum is defined by its frequency. The frequency determines the consistency (a degree of density, firmness, viscosity, etc) of the quantum. A quantum of very high consistency is called mass. These very high consistency quanta form the nucleus of the atom.
As the frequency of quantum decreases its wavelength increases. The wavelength determines the size of the quantum. In other words, as the consistency of the quantum decreases, its size increases. For example, an electron has much less consistency (is much softer) and also has much bigger in size than a nucleon in the nucleus. This is evident from the hydrogen atom, where its single electron is the size of the atom.
There is a whole spectrum of quantum from neutron in the nucleus of the atom to the photon of light. On the spectrum, the frequency (consistency) is continually decreasing and, consequently, the wavelength (size) is continually increasing. The size of the photon is large enough to explain the phenomenon of “quantum entanglement” over a distance of miles.
A quantum does not have a point location. That is a fixed idea among some people, which is a holdover from classical mechanics. In classical mechanics, the location of a matter particle was approximated by a point because of the property of center of mass. This center of mass property no longer applies in quantum domain.
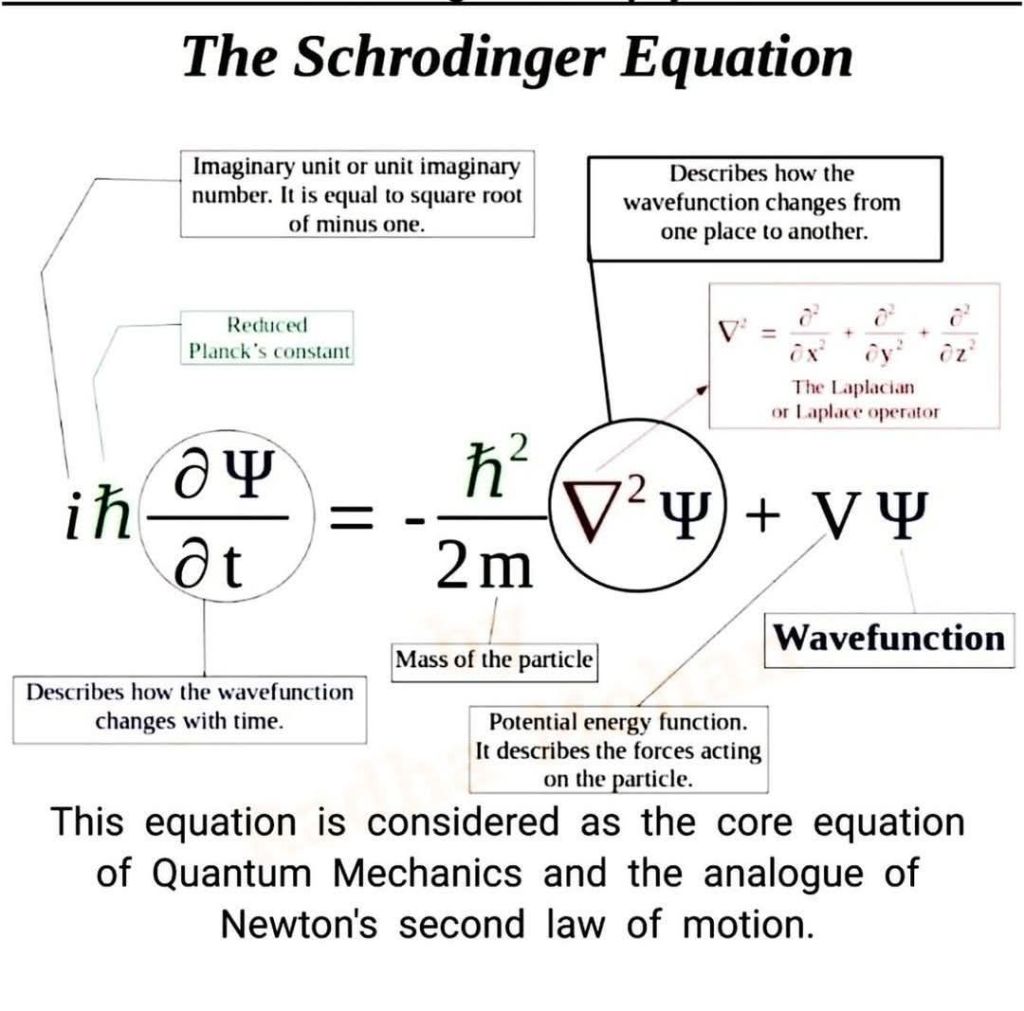
The Schrödinger Equation, which is the core equation of quantum mechanics, is basically an analogue of Newton’s second law of motion. This equation is solved for the wave function that includes information about the quantum, such as, position, momentum, spin, the form and its evolution. The time-independent Schrödinger equation describes a fixed pattern among quanta, such as that of electrons in a stable atom.
The wave-particle property has to do with the size and consistency of a quantum. The Planck’s constant in the Schrödinger Equation links the consistency of quantum to its frequency, and the momentum of the particle to its wavelength. The imaginary unit enables accurate descriptions of wave functions, probability conservation, and interference effects. The mass affects the spatial spread, radiation quantization, curvature and oscillations of the wave function.
Thus, on the spectrum of the quantum, as the frequency (consistency or rigidity) of the quantum decreases, and its wavelength (the size or spatial spread) increases, the velocity (motion characteristic) of the quantum also increases.
.